Introduction: Mutations in RAS-pathway genes ( NRAS, KRAS, CBL) are amongst the most common somatic mutations in cancer and historically resistant to most therapies. Targeted therapies that impact the RAS-pathway constitute a major unmet need in oncology. The proliferative form of chronic myelomonocytic leukemia (CMML) is commonly associated with RAS-pathway mutations and has a high propensity to develop into acute myeloid leukemia. In pre-clinical models, Lenzilumab (LENZ; Humanigen, Inc., Short Hills, NJ), a proprietary Humaneered ® first-in-class monoclonal antibody with best-in-class off-rate and affinity that neutralizes GM-CSF, resulted in a reduction of colony numbers and viability of CMML cells, with the greatest sensitivity in cells possessing RAS-pathway ( NRAS/ KRAS/ CBL) mutations, suggesting benefit in the targeted treatment of CMML. The PREcision Approach to CHronic Myelomonocytic Leukemia (PREACH-M) trial assesses the efficacy of LENZ, in addition to azacytidine (AZA), in CMML subjects with RAS-pathway mutations and high dose sodium ascorbate (ASC) and AZA in CMML subjects without RAS-pathway mutations. Interim data from 11 subjects with RAS-pathway ( NRAS/ KRAS/ CBL) mutations in PREACH-M receiving LENZ/AZA demonstrate reductions in circulating GM-CSF and CRP with 8 subjects achieving complete response or optimal marrow response. This report describes improvements in variant allele frequencies (VAF) for RAS-pathway mutations and hematologic improvements associated with LENZ/AZA treatment in CMML.
Methods: PREACH-M is a Phase 2/3 non-randomized, open-label trial in 72 subjects, aged at least 18 years, with newly diagnosed CMML based on the WHO 2016 criteria; and RAS-pathway mutations at a variant allele frequency ≥3%. Subjects received 24 cycles (every 28 days) of AZA (SC; 75 mg/m 2 for 7 days) and LENZ (IV; 552 mg; d1 & d15 of cycle 1 and d1 only for all subsequent cycles). Subjects without RAS-pathway mutations received the same AZA regimen and sodium ascorbate (IV; 30g for 7 days (15g for 1st dose only; 30g thereafter if no evidence of tumor lysis syndrome); PO; 1.1g on all other days). VAF was determined from bone marrow mononuclear cells using a 41-myeloid panel using Illumina Hi-Seq with a depth of 1000x performed on bone marrow aspirates obtained at baseline and Day 1 of treatment cycles 4, 7, and 12.
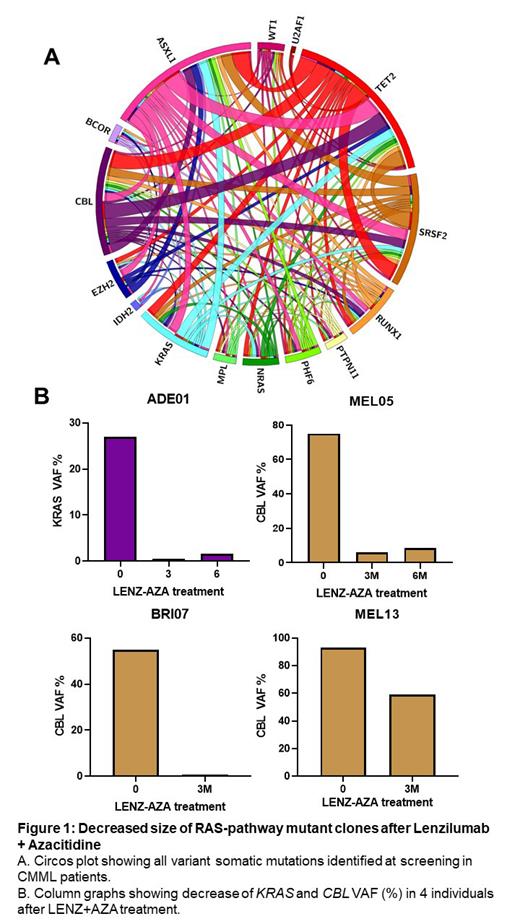
Results: As of July 2023, 11 subjects completed at least 3 months of LENZ/AZA treatments and follow-up sequencing data were available for 10 subjects. Five of 10 subjects showed >10% decrease in VAF for at least one mutation detected at baseline, including 5 decreased alleles in the RAS-pathway ( KRAS, CBL) out of 22 total VAF mutation responses. CBL mutations showed the largest change with a decrease in >50% VAF in three subjects. Decreases in RAS-pathway clones by LENZ/AZA were durable at 6 months. No decreases in VAF for any mutations were observed in 2 evaluable subjects following ASC/AZA treatment. Following 3 months LENZ/AZA treatment, blood monocyte count improved from a baseline of 11.0 ± 6.0 x 10 9/L to 2.0 ± 1.0 x 10 9/L (p=0.030). Blast differential improved from 10.5 ± 5.0% to 4.0 ± 3.5% (p=0.038). Platelet count increased from 90.0 ± 50.0 x 10 9/L to 150.0 ± 60.0 x 10 9/L (p=0.010). Statistical improvements remained durable for at least 6 (n=6) and 12 (n=3) months. Hemoglobin concentration increased 100.0 ± 22.0 g/L to 115.0 ± 10.0 g/L at 3 months (p=NS, n=11) and progressively increased to 122.0 ± 10.0 g/L at 12 months (p=0.024, n=3). Spleen length decreased from 15.0 ± 4.0 cm to 13.0 ± 4.0 cm (p=0.03) and decreased further to 11.0 ± 2.0 cm in subjects who received 12 months of treatment (n=3). No evidence of disease progression or relapse was observed in any subject.
Conclusion: In 11 evaluable subjects with proliferative CMML and RAS-pathway mutations, GM-CSF neutralization with LENZ in addition to AZA standard of care, resulted in significant decreases in the proportion of KRAS and CBL mutant leukemic cells, accompanied by clinically significant hematologic improvements and a reduction in splenomegaly. Lenzilumab may have efficacy in preventing outgrowth of RAS-pathway mutations, specifically KRAS and CBL, in the context of CMML and other myeloid malignancies.
OffLabel Disclosure:
Ross:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Menarini: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Honoraria, Research Funding. Fong:BMS: Honoraria; Jazz: Honoraria; Otsuka: Honoraria; Novartis: Honoraria; Pfizer: Honoraria, Speakers Bureau; Servier: Honoraria, Speakers Bureau; BeiGene: Honoraria; Amgen: Honoraria; AbbVie: Honoraria, Speakers Bureau; Astellas: Honoraria. Yong:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Celgene: Research Funding. Yeung:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hughes:Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Terns Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Enliven: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Lenzilumab is a humanized monoclonal antibody that neutralises granulocyte-macrophage colony-stimulating-factor. The drug is being used to treat patients with high risk chronic myelomonocytic leukaemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal